Clinical Trials and Research
The US Food and Drug Administration (FDA) has approved inotersen (Tegsedi, Akcea Therapeutics and Ionis Pharmaceuticals) for the treatment of polyneuropathy (PN) in…
A new hereditary ATTR (hATTR) amyloidosis treatment, inotersen, an investigational antisense drug, may not be far from a US Food and Drug Administration (FDA)…
Amyloidosis is a rare condition that used to almost always be fatal, but doctors are finally making progress in treating it.
"I had so many symptoms, and I really…
Updated research regarding the ANDROMEDA study presented at the 2018 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, Illinois, indicated the…
Amyloidosis is a disorder in which certain proteins abnormally change their shape in a process called "misfolding." The misfolded proteins accumulate together and…
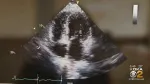
Cardiac amyloidosis, traditionally considered a rare and dire disease, is receiving increasing attention as sophisticated diagnostics are identifying more patients…
Although most patients with familial amyloid polyneuropathy (FAP) who undergo liver transplant will require a pacemaker, implanting the device before the transplant…
AL amyloidosis is a rare condition caused when bone marrow produces abnormal antibodies that can't be broken down and are deposited in tissues, interfering with…
Once used to diagnose myocardial infarction, technetium-99m pyrophosphate (Tc 99m PYP) imaging may be reborn as an alternative to biopsy for diagnosing cardiac…
The FDA announced that it has approved patisiran infusion for the treatment of patients with peripheral nerve disease caused by hereditary transthyretin-mediated…